Battery Technology and Maintenance

If you read my last blog about my UPS battery woes and it interested you, then this one might help you further.
Batteries are a horrible technology in many respects. They’re expensive, damaging to the environment and incredibly inefficient. Unfortunately we have nothing better so we have to make do until another technology is found.
When it comes to large batteries, you’ll generally only find one type of battery – lead acid. They come in several forms:
- FLA (Flooded Lead Acid) – The type found in cars
- AGM (Absorbed Glass Mat) – The type found in UPS’s and alarm systems
- SLA (Sealed Lead Acid, commonly mislabeled and are really AGM) – Also found in UPS’s and alarm systems
While not the best at energy storage compared to Lithium Ion/Polymer, they are much cheaper and store close to the same amount of energy too.
Taking care of these batteries is important (as with any battery actually) because of the way in which they work.
A lead acid battery is comprised of lead plates and an electrolyte solution of sulfuric acid. This is how the battery holds it’s energy. During normal use, the energy will be released and stored via a chemical reaction. This reaction heats the electrolyte and over time this solution evaporates. If this happens too much and the lead plates become exposed and/or dry, the capacity of the battery will drop. Generally “going dry” is what kills a lot of mis-used batteries.
But this isn’t all, there is also another process called “sulphation”. This occurs when the battery is discharged and sulphur builds up on the lead plates. This inhibits it’s ability to hold and release energy. This also kills batteries when mis-used, especially when left discharged. This is common in cars and other vehicles, especially in winter when the battery is put under more strain.
Battery Charging
Now the problem I had was mainly the cells going dry through what is known as “float charging”. This is when the battery is held at a voltage above it’s natural storage voltage to keep the charge level at 100%. This is constantly putting some energy into the battery so it warms up, and the electrolyte dries off. This is what killed my UPS batteries, because the UPS was in essence over-charging the battery.
How can this be avoided? Don’t keep it on float of course, or if you do, do it at a reduced voltage. My Belkin UPS’s keep a float charge of about 13.3-13.6v. A standard 12v battery will sit at about 12.8-13.3v when fully charged, so if you can keep it around that voltage, you should extend the life of the battery. However, if you don’t bring a 12v battery up to 14.0-14.5v when charging, it will never fully charge and so you will end up sulphating the plates quicker. It’s a tricky situation, but it can be avoided.
The answer is 2 or 3 stage charging. 3 stage charging involves:
- Bulk phase – Charging the battery at a constant current (generally 10-30% of the battery capacity, referred to as “C”). For example, 10Ah battery holds 10,000mAh of capacity, so you would charge it at 1000-3000mA or 1-3A.
- Absorption phase – Charging the battery at constant voltage (typically 14.0-14.5v for a 12v battery) until the current drops to about 1% of C. For a 10Ah battery this would be 100mA or 0.1A.
- Trickle phase – Holding the battery at a reduced constant voltage (the tricky part is what voltage to use here).
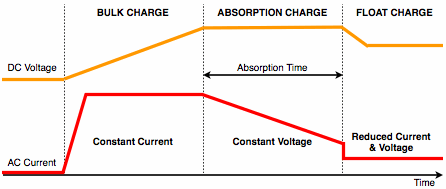
Image Credit: http://www.infinitumstore.my/2009/07/3-stage-charging-process/
2 stage charging is the same as above but without the trickle charge phase.
My Belkin UPS appears to do 3 stage charging, and it’s trickle voltage is around 13.3-13.6v. The difference with mine is it’s not constant voltage but rather it pulses on and off. Pulsing has been tested but there is no definitive proof it helps over the long run.
The better way to do this would be to do 2 stage charging, then cut off the charging completely until the voltage drops below a set level. Then you begin the process over again. This is the better way as it lets the battery rest with full capacity, without charging to make the electrolyte evaporate off.
I don’t know why more systems don’t do this. I imagine it’s for simplicity in design, otherwise they would need to incorporate a fully battery monitoring system which would cost more money. Who knows, but it makes sense to cut it off after it’s charged. This is especially important for lithium batteries like those in mobile phones. If you over-charge these (continue to charge when full), they can explode! So why do it with lead acids of any kind?
What about discharging?
Now that you know about charging, what about safe discharge levels? This is one that has plagued many people. Generally voltage would be used, wouldn’t you think? Well no, because lead acid batteries charge/discharge level cannot be determined without use of a hydrometer. This is a device that you use to sample the electrolyte from the battery and uses floats to determine the “specific gravity” of the electrolyte. I won’t go into how this works, but it’s something you can only do manually. Voltage on any battery is not a charge level indicator, you can only use it as a dummy guide and it will often fail you.
That said, you should be aware of safe limits that lead acid batteries work to. Here is a handy table to explain it to you.
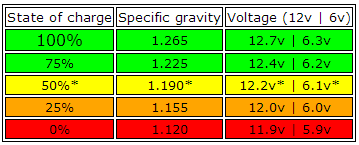
As a general rule, the battery should never be discharged past 12v when “resting”. Resting means it has been sat for more than 4 hours with zero load connected to it. With a load connected, you should never discharge below 10.5v. If you continue to discharge it, you risk warping the lead plates, and doing so can create a short circuit which will ruin your battery.This is a very basic table of charge level based on voltage and what the specific gravity is expected to be. Going in the standard good to bad colours, you can see that the battery capacity is only good above 50%. This is because below this, sulphation occurs much more quickly. On a totally flat battery this can be as quickly as a day or two.
You should also ALWAYS charge a lead acid battery after it has been used. Even if it was just a few percent, because any discharge speeds up plate sulphation.
This is something I didn’t take into account when building my camping power box. My LED volt meter range goes from 10.7v to 12.5v when really it should have been 12.0 to 12.6v. However, that said, now that I know it’s wrong I can use my own knowledge to determine when to stop using it and recharge it. The added range allows me to see when it’s time to stop using it under load as well.
Hopefully you have learned something from this and it’s a reminder for me if nothing else.
